Dental
Allografts for Dental Procedures
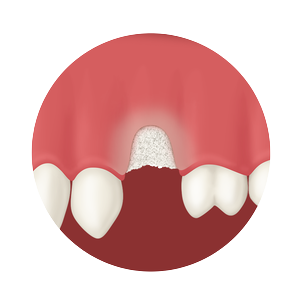
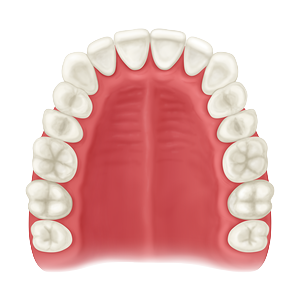
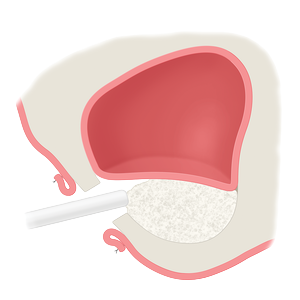
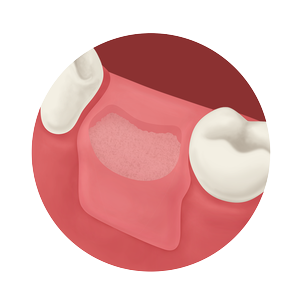
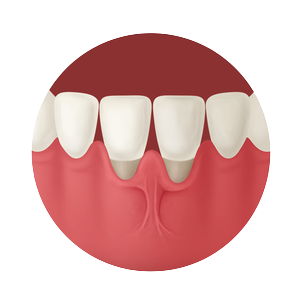
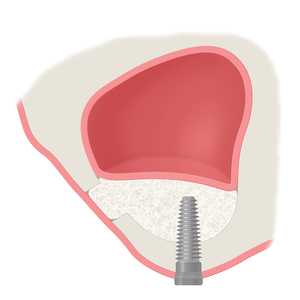
Whether treating periodontal disease, socket grafting, ridge augmentation or sinus grafting, our growing portfolio of dental solutions provides convenient, safe and proven solutions for healing.
The convenience and availability of LifeNet Health’s structural and soft tissue dental allografts is unmatched, and because of our industry-leading technologies, they carry the assurance of medical device-grade sterility to help protect patient safety.