FlexiGraft® DualLink®
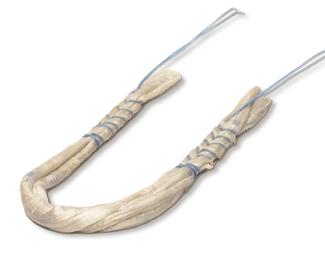
FlexiGraft®
FlexiGraft DualLink is a pre‑sutured tendon construct that is indicated for soft tissue
approximation and/or ligation. It can be used for ACL and PCL Reconstruction procedures.
- Convenience: Pre-sized graft reduces prep time and saves the time required for autograft recovery. An out‑of‑the‑box option for OR efficiency.
- Consistency: Trained technicians assemble the allograft for consistency, removing the variability between surgical assistants or physician assistants preparing the tendon in the OR. Grafts are pre-sutured under tension and receive 15 - 20 lbs. of tension for 15 minutes after suturing.
- Appropriate Strength for ACL & PCL: Biomechanical testing demonstrates that DualLink pre-sutured tendons have greater ultimate load than the native ACL.1
- Compatible: Construct was designed to be used with the Arthrex TightRope® RT sutures.
- Patient-Friendly: Construct eliminates the donor site morbidity and associated pain from autograft harvest. This makes the procedure less invasive and potentially decreases OR time. Less OR time can mean less time under anesthesia and less tourniquet time.
- Sterile: DualLink is sterilized using proprietary and patented Allowash XG® technology for patient safety. This provides a sterility assurance level (SAL) of 10‑6, without compromising the construct’s inherent biomechanical properties.
This product is not available in all markets.
Clinical Application
- ACL reconstruction
- PCL reconstruction
| Description | Sizing | |
|---|---|---|
| FDL | Length is measured under hand tension in 5 mm increments. Diameter is measured by pulling the folded construct through a sizing block with light force starting with the largest channel and sequentially working down until the tendon no longer passes through. The smallest channel the tendon can pass through is the recorded diameter. | 9.0 - 11.0 mm D / 175 - 260 mm L |
References
1. Hutchens et al. Pre-sutured Single Fold Tendon Design Qualification Biomechanical Testing. LifeNet Health. 2022; ES-22-026.
