FlexiGraft® GraftLink®
FlexiGRAFT®
FlexiGraft® GraftLink®
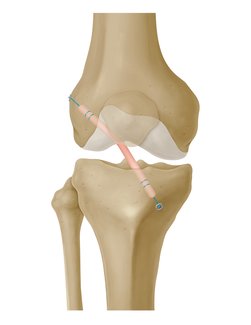
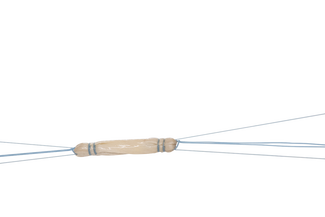
GraftLink is a pre‑sutured tendon construct that is indicated for soft tissue approximation and/ or ligation. It can be used for ACL reconstruction procedures.
This product is not available in all markets
- Convenience: No prep time or timely harvest of autograft required, no training for staff necessary. An out-of-the-box option for OR efficiency.
- Consistency: Trained technicians suturing graft for construct consistency. Removes the variability between surgical assistants or physician assistants preparing the tendon.
- Appropriate Strength for ACL: Biomechanical testing demonstrated that GraftLink pre-sutured tendons have greater ultimate load than unstitched tendons. Testing also showed that tibial side fixation was stronger than traditional interference screw.1
- Compatible: Construct was designed by Arthrex® to be used with their GraftLink All-Inside® Technique. Specialized tools, fixation, and guides have already been developed and are in use by surgeons today.
- Patient-Friendly: Construct eliminates donor site morbidity and associated pain from autograft harvest. This makes the procedure less invasive and potentially decreases OR time. Less OR time can mean less time under anesthesia and less tourniquet time.
- Sterile: GraftLink is sterilized using proprietary and patented Allowash XG® technology. This provides a sterility assurance level (SAL) of 10-6, without compromising the construct’s inherent biomechanical properties.
Clinical Application
- ACL Reconstruction
| Description | Sizing | |
|---|---|---|
| FGL | GraftLink® | L = 60 - 80 mm; D = 7.5 - 10.5 mm |
References
- Data on file at Arthrex, Inc.
