ADVANCED FIBER TECHNOLOGY
LifeNet Health’s fiber technology is an innovation that provides a solution to fusion by harnessing nature’s time-tested patterns and strategies.
Hospitable Scaffold with Fiber Technology
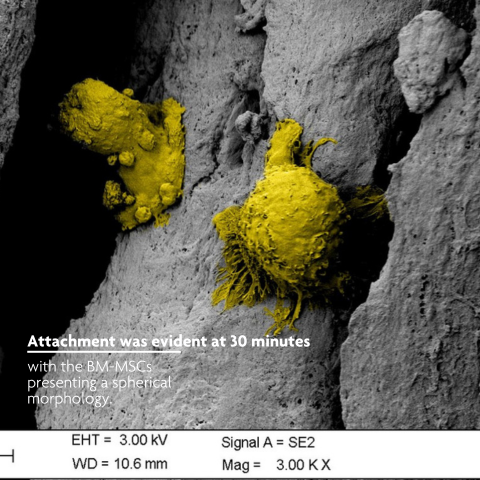
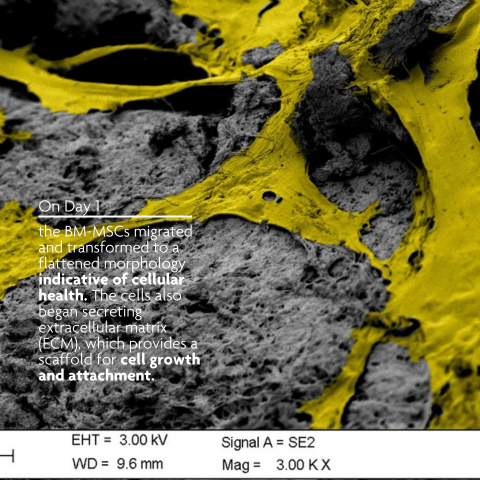
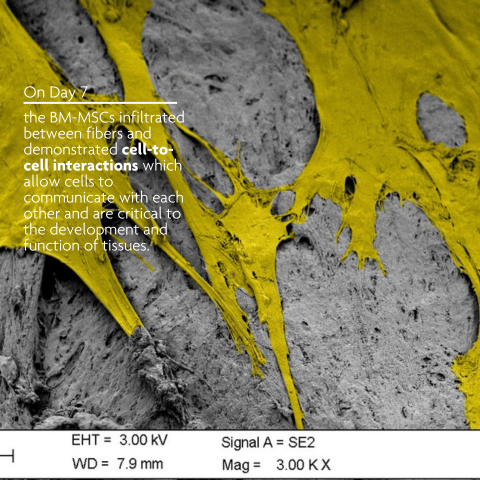
LifeNet Health has developed a demineralized bone matrix (DBM) that is comprised of interlocking fibers. The long, rough cortical fiber surface of PliaFX demineralized bone fibers provide many contact points for cellular attachment, and the interconnected fibers allow the cells to spread out and make cell-to-cell connections. PliaFX contains interconnected, optimally demineralized bone fibers which provide the osteoconductive and osteoinductive properties necessary to support cellular proliferation and bone formation.1,2,3 †
PliaFX encourages cellular attachment, migration and infiltration2,3 †
These interlocking fibers become moldable upon rehydration, conform to the surgical site, and resist migration. PliaFX Prime offers flexibility in rehydration, allowing the option to use soluble antibiotic solution.1,2,3 †
Precise Demineralization Process for Regeneration
- PliaFx Prime is optimally demineralized using LifeNet Health’s proprietary technology to expose growth factors such as bone morphogenic proteins (BMPs), which can recruit host cells to the implant site and stimulate bone-forming activity.1,2,3 †
- Literature has shown that BMPs including BMP-2 & BMP-7 are important for bone healing as they are known for their ability to stimulate differentiation of MSCs to osteochondroblastic lineage and are important factors necessary for bone healing and regeneration.2 †
- Angiogenic growth factors, VEGF and angiogenin, were detected in PliaFX Prime samples (see table). These results demonstrate that PliaFX Prime shows angiogenic potential.1,2,3 †
Safety Profile
LifeNet Health prides itself on our safety record over the last 40+ years. We hold the longest continuous accreditation from the American Association of Tissue Banks (AATB) and have a comprehensive range of measures in place to validate the safety of our allograft bio-implants; this includes stringent donor screening methods and release criteria. To obtain suitable donors, we maintain an extensive network of recovery partners. Additionally, we are a leading, federally designated Organ Procurement Organization. We only accept donors from federally designated Organ Procurement Organizations and qualified tissue recovery partners. These partners are regularly audited to document that their recovery process meets current FDA regulations, AATB standards, and our own stringent guidelines. It is important to KNOW your tissue provider.
References
1. Data on file LifeNet Health 63-0252-03.03.
2. McLean JB, Carter N, Sohoni P, Moore MA. Cell Attachment and Osteoinductive Properties of Tissue Engineered, Demineralized Bone Fibers for Bone Void Filling Applications. Clinical Implementation of Bone Regeneration and Maintenance. 2019.
3. DePuy Synthes. PliaFX® Prime – Moldable Demineralized Fibers White Paper. 2022.AMP Document #US_DPS_SPNE_170825.
† Pre-clinical test data/results may not necessarily be indicative of human clinical performance (or outcomes).