Sports Medicine
As the world’s leading provider of sports medicine allografts, with effective solutions for procedures ranging from ACL repairs to complex reconstructions, LifeNet Health’s high-quality allografts are carefully processed to retain the characteristics required to perform successful sports medicine surgeries.

ArthroFlex®
ArthroFlex is an acellular dermal matrix (ADM) intended for supplemental support and covering for soft tissue repair. ArthroFlex is frequently used for procedures such as superior capsular reconstruction, rotator cuff repair, and achilles tendon repair due to its strength, sterility, and convenience.
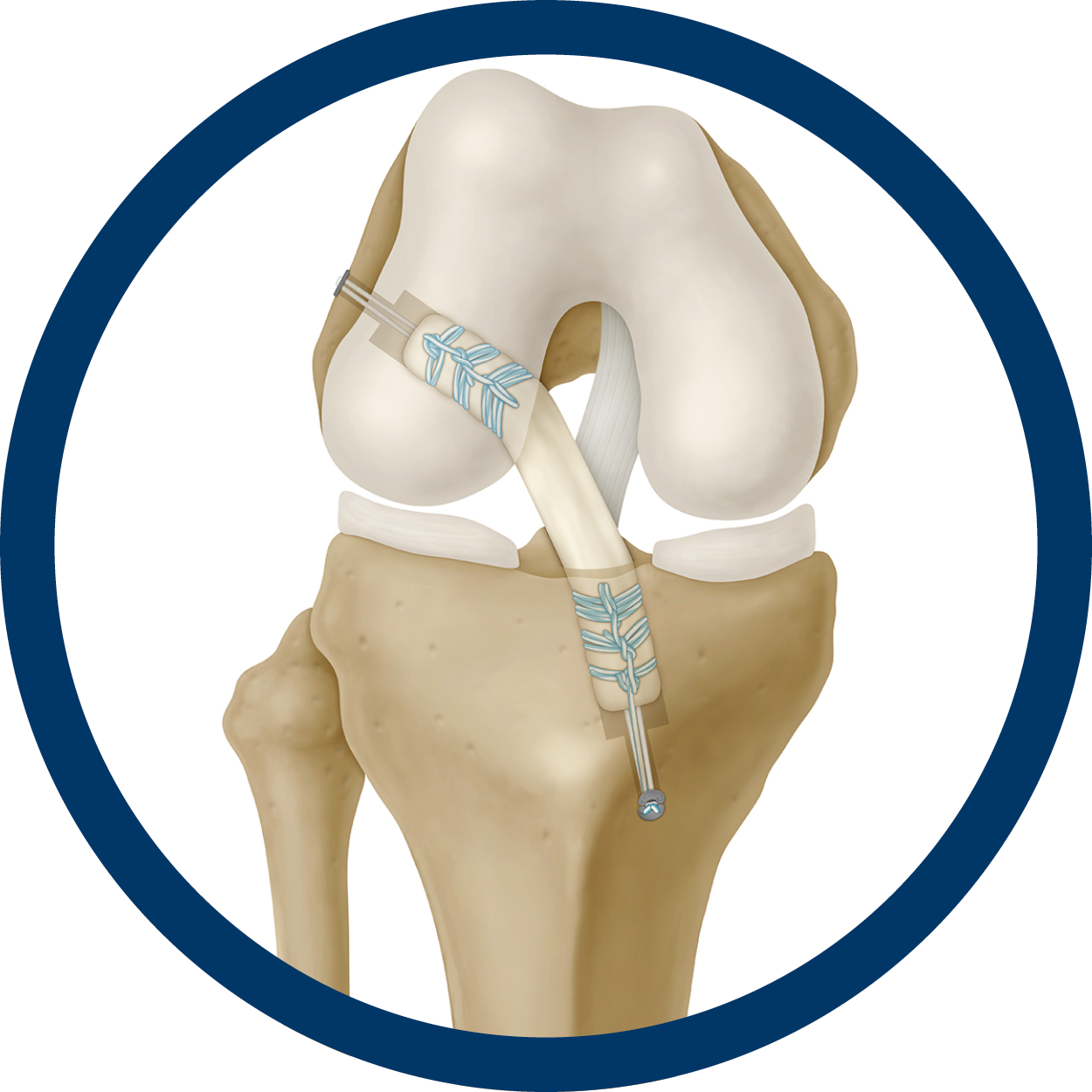
FlexiGraft®
FlexiGraft tendons, pre-sutured tendons, cancellous plugs, revision dowels, and meniscus are proven to be clinically effective for numerous sports medicine procedures. This includes, but is not limited to, ACL and PCL repair, MCL, LCL & MPFL repairs, multiligament repairs, lateral ankle reconstruction, and meniscus transplantation.
Fresh Osteochondral Allografts
Our Fresh Osteochondral Allografts offer solutions for repair of various articular cartilage and the subchondral bone defects. Stringent donor screening, processing, and preservation procedures ensure safe and high quality allografts with viable hyaline cartilage.
Note: Not all bio-implants are available for distribution in every country.
Download the full catalog, click here.

.png)
