FlexiGraft® Lateral Ankle
FlexiGRAFT®
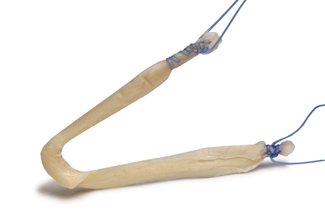
FlexiGraft® Pre-Sutured Lateral Ankle Tendon Constructs
These Lateral Ankle Tendon Constructs offer out-of-the-box convenience, eliminate donor site morbidity and pain issues due to autograft harvests and are medical device grade sterile (SAL 10-6)
*This graft may not be available in all countries.
- Convenience: No prep time or time-consuming harvest of autograft required, no training for staff necessary. An out-of-the-box option for OR efficiency
- Pre-Sized: Construct designed to a diameter of 4 to 5 mm and length of 150 to 160 mm – eliminating the need for trimming by the surgeon’s staff
- Consistency: Trained technicians suturing graft for construct consistency. Removes the variability between surgical assistants or physician assistants preparing the tendon
- Patient-Friendly: Construct eliminates donor site morbidity and associated pain from autograft harvest. This makes the procedure less invasive and likely decreases OR time. Less OR time can mean less time under anesthesia and less tourniquet time
- Sterile: Pre-Sutured Lateral Ankle Tendon is sterilized using proprietary and patented Allowash XG® technology. This provides a sterility assurance level (SAL) of 10-6, without compromising the construct’s inherent biomechanical properties
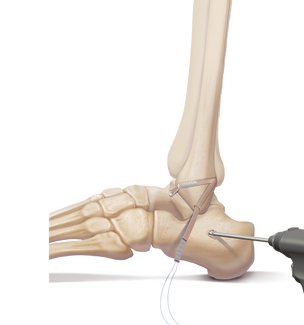
Clinical Application
Lateral Ankle Reconstruction
| Description | Sizing | |
|---|---|---|
| FPSST | Pre-Sutured Lateral AnkleTendon Length is measured to be 150 - 160 mm without tension. Diameter is measured single strand by pulling the construct through a tendon sizer with modest pressure. The recorded diameter is the smallest channel the tendon will pass through. | L = 155 mm +/- 5 mm; D = 4 - 5 mm |
