Vascular
AngioGraft® cryopreserved allograft vessels are the natural choice for managing vascular reconstruction. LifeNet Health grafts consistently perform as they should, allowing medical professionals to focus on the procedure, and patients to focus on healing.
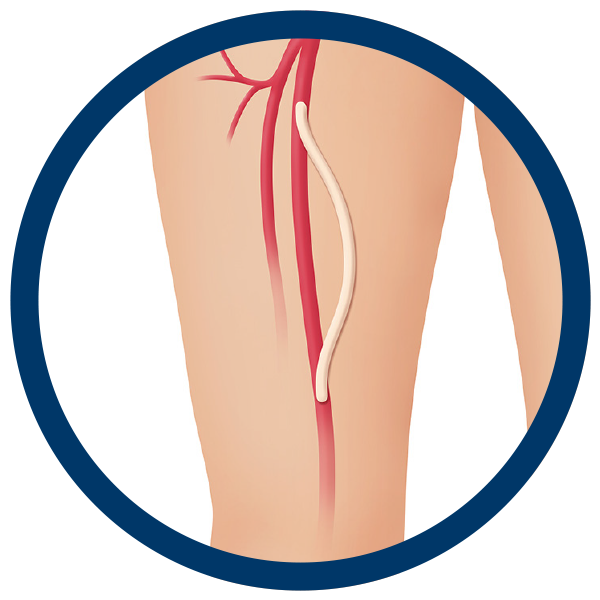
Peripheral Vascular Disease (PVD) and Critical Limb Ischemia (CLI)
Cryopreserved allografts provide a natural solution to help treat critical limb ischemia. Allografts closely resemble native tissue, making them compliant, flexible, and easy to handle. Allografts also are naturally resistant to infection, avoid donor site morbidity, and are an ideal conduit in cases where the use of autologous vessels is not possible.
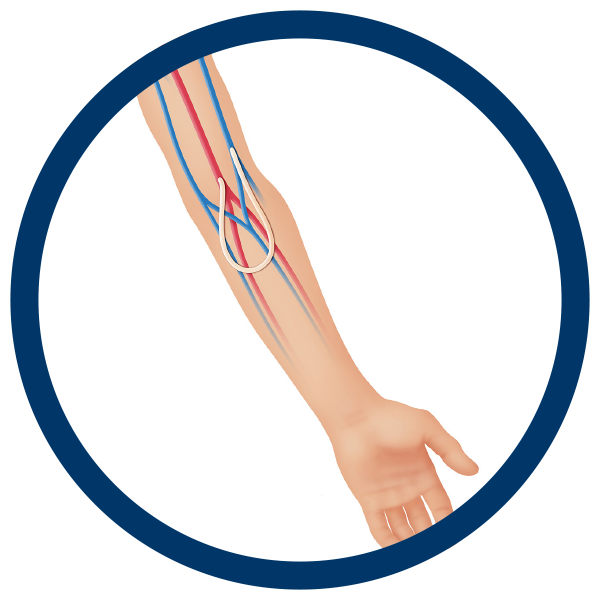
Arteriovenous (AV) Access
Following a failed AV fistula, cryopreserved femoral veins and arteries provide an ideal solution to AV access for dialysis. In cases of infection or in patients at a greater risk of infection, allografts can also provide a safer solution due to their natural resistance to infection.1,2,3
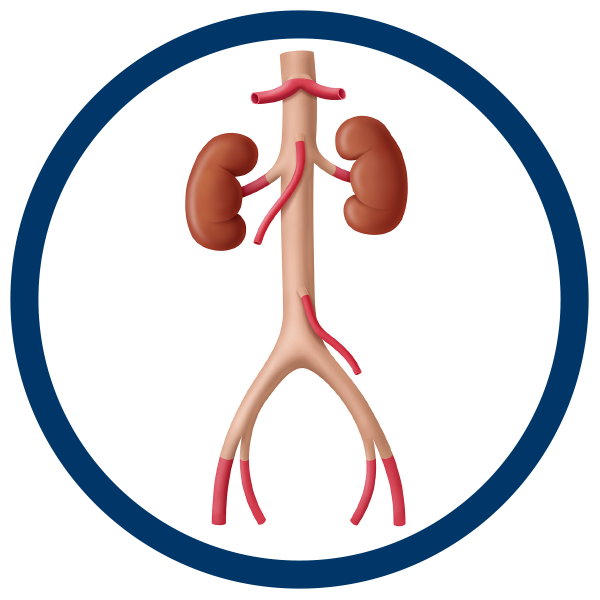
Aortoiliac Disease and Abdominal Aortic Aneurysm (AAA)
Aortoiliac disease is one of the most difficult-to-treat conditions that a surgeon faces. Whether due to a failed synthetic graft, an aortoenteric fistula, or a mycotic aneurysm, cryopreserved aortoiliac (AI) grafts provide an ideal solution for the treatment and care of patients with aortoiliac disease.
1. Vardanian et al. Arterial Allograft Allows In-line Reconstruction of Prosthetic Graft Infection with Low Recurrence Rate and Mortality. The American Surgeon October 2009 Vol. 75, No. 10: 1000-1003.
2. Madden et al. Experience with cryopreserved cadaveric femoral vein allografts used for hemodialysis access. Annals of Vascular Surgery 2004; 18: 453-458.
3. O’Banion et al. Cryopreserved saphenous vein as a last-ditch conduit for limb salvage. Journal of Vascular Surgery 2017, Volume 66, Number 3: 844-849.
