.png)
Soft-tissue grafts, such as dermis and cardiovascular tissue contain donor cells that can elicit an inflammatory reaction, limiting the effectiveness and long-term durability of the graft. Tissue treated with LifeNet Health's patented and validated Matracell technology process negates that risk by yielding a strong, acellular scaffold that facilitates cell proliferation, cell migration and vascularization.
Matracell technology has been shown to to decellularize dermis, resulting in >99 donor DNA removal in cardiac tissue. In fact, only tissue processed with Matracell technology meets the threshold of being truly decellularized, thereby minimizing the possibility of an immune response. Not all decellularization processes meet the definition. Evidence suggests that <50ng dsDNA per mg ECM dry weight of residual DNA satisfies the intent of decellularization.1
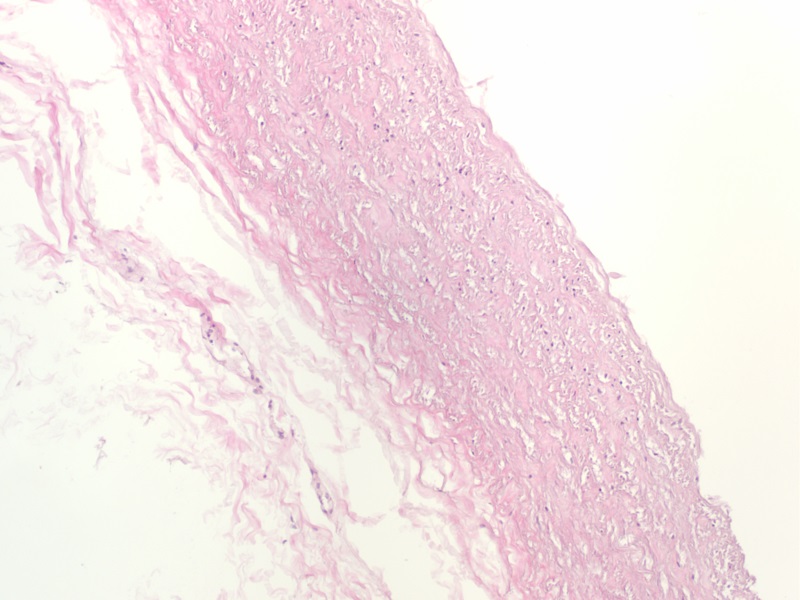
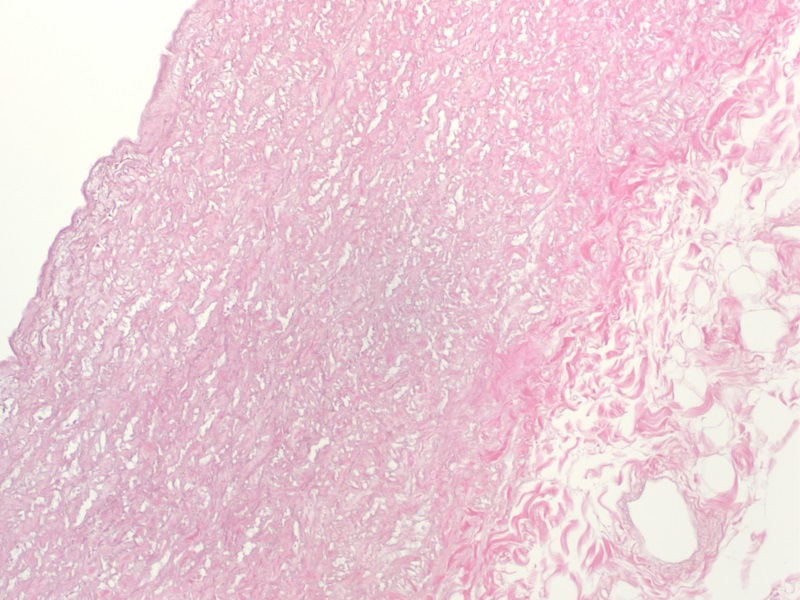
Histology Demonstrates the Thorough Removal of Cellular Content from Pulmonary Artery Tissue
H&E staining demonstrates that cellular content (Blue/Purple stain) is completely removed following decellularization, leaving behind only the components of the extracellular matrix (Pink stain).
.jpg)
Before
After
- Peer Reviewed Articles
- Prototype Anionic Detergent Technique Used to Decellularize Allograft Valve Conduits Evaluated in the Right Ventricular Outflow Tract in Sheep. J Heart Valve Dis. 2014
- From cadaver harvested homograft valves to tissue-engineered valve conduits. Progress in Pediatric Cardiology. 2006
- Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. The Journal of Thoracic and Cardiovascular Surgery. 2009
- Pulmonary Arterioplasty With Decellularized Allogeneic Patches. Ann Thorac Surg 2014
- Ovine panel reactive antibody assay of HLA responsivity to allograft bioengineered vascular scaffolds. Evolving Technology. 2005
- Recellularization of Decellularized Allograft Scaffolds in Ovine Great Vessel Reconstructions. Ann Thorac Surg. 2005
- Measurements of the Effects of Decellularization on Viscoelastic Properties of Tissues in Ovine, Baboon, and Human Heart Valves Tissue Eng Part A. 2012
- Organ engineering based on decellularized matrix scaffolds. Trends in Molecular Medicine. August 2011
- Viral Inactivation of Human Osteochondral Grafts with Methylene Blue and Light. Cartilage. 2014
- White Papers and Technical Papers
1. Crapo PM, Gilbert TW & Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011. April;32(12):3233-3243

