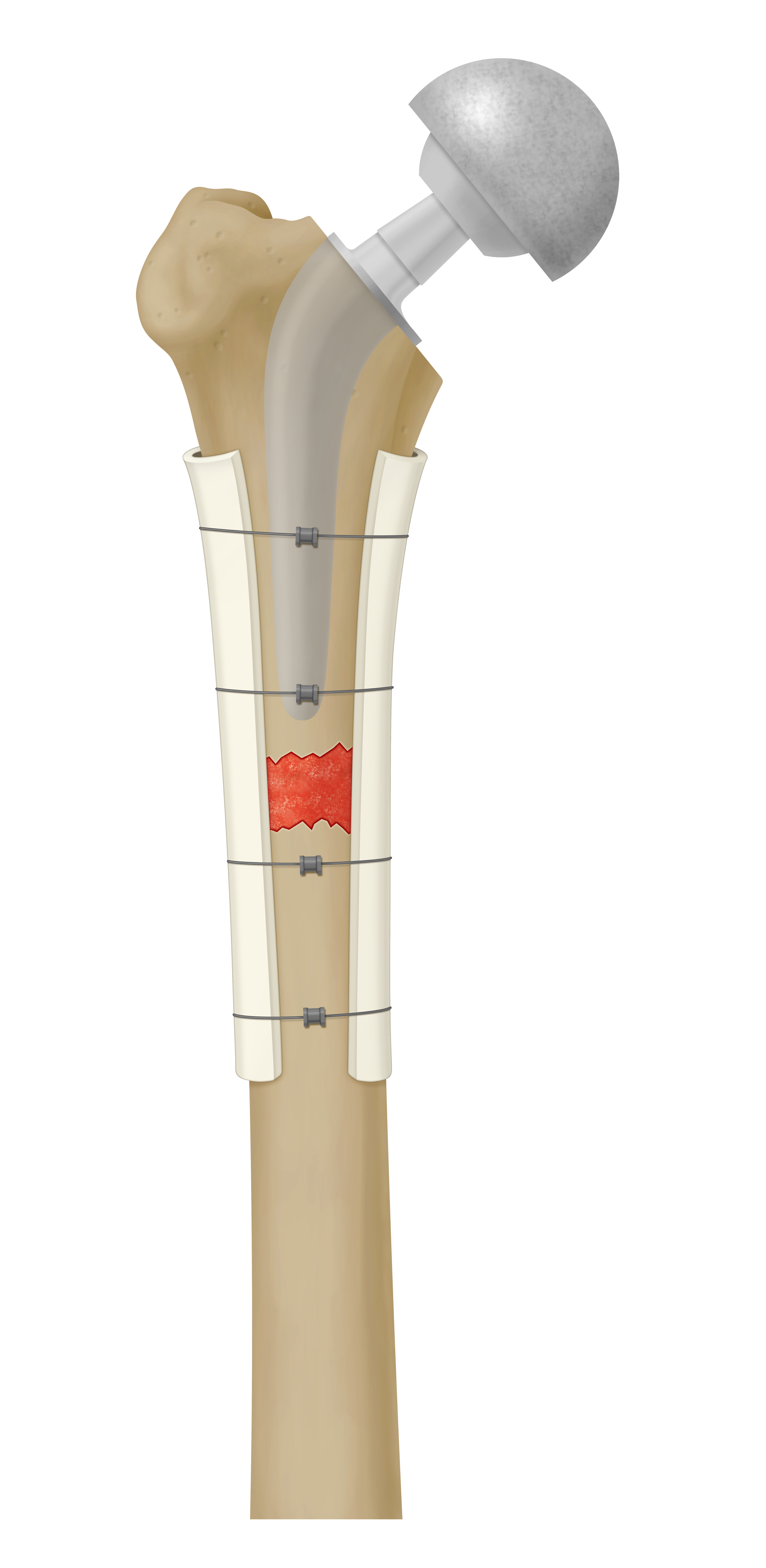
Our full line of allograft biologic implants provide surgeons with the tools they need to improve the lives of patients. From sports medicine to spine, from cardiac and vascular to skin and wound, from craniomaxillofacial to general orthopedics and trauma, our comprehensive portfolio provides surgeons - and their patients - with the right graft for the right procedure. Click on a product icon below for more information.
Our philosophy is simple: When partnering with an allograft supplier, we believe the decision should be based on the overall value clinicians and patients expect and deserve. At LifeNet Health, we deliver that value by excelling in six critical areas:
Since 1995, more than 13 million allografts processed using Allowash XG® sterilization technology have been distributed by LifeNet Health, with no disease transmission. By using a network of qualified and trained recovery partners, one of the most stringent screening and recovery protocols, patented tissue-cleaning and sterilization processes, and a highly controlled processing environment, we counter the risk of disease transmission at every step.
LifeNet Health’s controlled processing environment is designed to ensure tissue quality and safety. Through the consistent application of quality systems, quality control, and design control processes, LifeNet Health tissue graft are designed and manufactured to ensure highest possible quality. Year after year, this dedication to quality is validated internally and vetted by your peers as well as government and industry regulators. LifeNet Health holds the longest running current accreditation by the American Association of Tissue Banks (AATB). Our certifications and memberships include AATB, CLIA (Clinical Laboratory Improvement Amendments), FDA registration, ISO 13485 (Standard for medical devices) and UNOS (United Network of Organ Sharing).
In keeping with the mission of saving lives, restoring health and giving hope, LifeNet Health’s focus on research and development has resulted in many U.S. issued patents. As part of its commitment to advancing tissue grafts and regenerative medicine, LifeNet Health has developed key innovations such as proprietary and patented cleaning, demineralization, and composite allograft technologies. A leader in its field, LifeNet Health continually works on innovations to advance tissue grafts safety and efficacy.
Customer service is available from 7am to 7pm EST, and on-call support is provided 24 hours a day for emergencies. LifeNet Health offers a flexible return policy, free shipping and Strategic Account Mana to consult with implanting surgeons and facilities.
With LifeNet Health as your primary bio-implant supplier, you are investing in the best possible value to ensure the well-being of your patients and the reputation of your hospital.
Our extensive portfolio of implants consistently performs at the highest level because LifeNet Health has invested considerable resources performing multiple clinical studies to ensure your patients outcomes are positive.
LifeNet Health offers you a partner with bi-coastal production facilities - ensuring a dedicated supply of much needed allograft products to your facility. We are working towards being able to produce and process our entire extensive portfolio at each facility, which supports nearly every surgical discipline.