Available in three configurations to meet a
variety of clinical needs.

PliaFX® Prime
Moldable Demineralized Fibers
PliaFX® Flo
Flowable Demineralized Fibers
PliaFX® Pak
Packable Demineralized Fibers with Chips
Available Applications
| 100% bone | |||
|---|---|---|---|
| Customizable Easily mixes with biomaterials such as autograft or allograft4,5,7,8 | |||
| Resists Migration Interlocking fibers allow graft to remain intact and in place | |||
| Convenience Ambient storage and no rehydration required10,2 | |||
| Osteoconductive Large surface area and interconnected network of fibers provide a scaffold that promotes cell attachment and spreading1† | |||
| New Bone Formation Potential Fibers were readily mineralized as early as 6 weeks when cultured in vivo indicating graft factors can promote bone-forming cells to mineralize the graft given the appropriate microenvironment4† | |||
| Hemostatic Facilitates coagulation to stop bleeding4† | |||
| Precise Delivery Packaged in a sterile syringe, allowing delivery directly to the surgical site3 |
Innovative Surface Technology
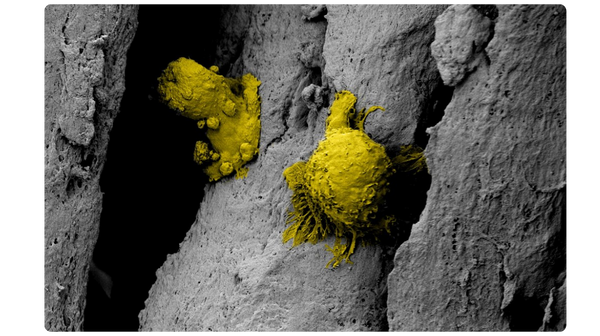
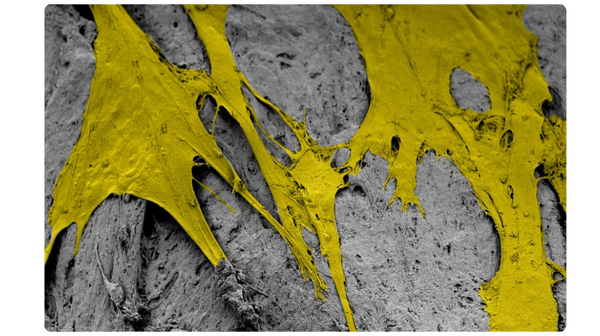
The unique surface characteristics of PliaFX fibers set the standard for osseointegration.4-6 With a large surface area and well-structured demineralized cortical fibers, PliaFX provides a solid scaffold that fosters cellular viability and proliferation.4† Our proprietary processing method creates favorable porosity and microhook protrusions, improving the graft's osteoconductivity.4,10†
| 
|
Scanning electron microscopy (SEM) at 3000x magnification. Images were pseudo colored in Adobe Photoshop to distinguish the cells (in yellow) from the fibers.
Optimized Handling
The interlocking fiber microhooks in PliaFX grafts provide optimized handling characteristics.4,5,7,8† The fibers interlock to provide a moldable, intact graft that easily transfers to the surgical site, conforms to the surgical site, and resists migration.4
Other characteristics include:

|
|

|
|
Advanced Processing
Utilizing PAD® technology, PliaFX fibers achieve optimal osteoinductive potential to support bone healing.1-4† This proprietary process ensures that our grafts maintain an optimal residual calcium level of 2%, preserving their natural osteoconductive and osteoinductive properties.1-4†
Uncompromised Safety
All PliaFX grafts are sterilized using Allowash XG® technology, providing medical device-grade sterility while preserving the biochemical and biomechanical properties of the graft.4,10† Since 1995, LifeNet Health has processed over 13 million grafts with this technology, boasting no incidents of disease transmission.10
Clinician Perspectives and Case Studies
References
- Zhang Q, Jing D, Zhang Y, Miron RJ. Int J Oral Maxillofac Implants. 2018 May/June; 33(3):645–652. PMID: 29420674 Osteoinductive potential of 4 commonly employed bone grafts
- Herold RW, Pashley DH, Cuenin MF, et al. The effects of Varying degrees of Allograft Decalcification on Cultured Porcine Osteoclast cells. J Periodontol. 2002 Feb; 73(2):213–9
- Mott DA, Mailhot J, Cuenin MF, et al. Enhancement of osteoblast proliferation in vitro by selective enrichment of demineralized freeze-dried bone allograft with specific growth factors. J Oral Implantol. 2002; 28(2):57–66
- McLean JB, Carter N, Sohoni P, Moore MA. Cell attachment and osteoinductive properties of tissue engineered, demineralized bone fibers for bone void filling applications. Clinical Implementation of Bone Regeneration and Maintenance, IntechOpen. 2021.
- Rodriguez RU, Kemper N, Breathwaite E, et al. Demineralized bone matrix fibers formable as general and custom 3D printed mold-based implants for promoting bone regeneration. Biofabrication. 2016;8(3):035007.
- Boyan BD, Ranly DM, McMillan J, et al. Osteoinductive Ability of Human Allograft Formulations. J Periodontol. September 2006.
- Dr. Byron Branch video, https://youtu.be/MVHRnkOO3lI
- Dr. Richard Yoon video, https://youtu.be/B1cOj1Yy4nA
- Eisenlohr LM. Allograft Tissue Sterilization Using Allowash XG®. Bio-Implants Brief. 2007. 68-0089.
- Data on file. LifeNet Health TR-0446.
- PliaFX Prime Instructions for Use (63-0252-03)
- PliaFX Flo Instructions for Use (63-0411)
† Pre-clinical test data/results may not necessarily be indicative of human clinical performance (or outcomes). Important Information: Prior to use, refer to the instructions for use supplied with the device(s) for indications, contraindications, side effects, warnings and precautions.